The analysis of the Concinto clinical trial database
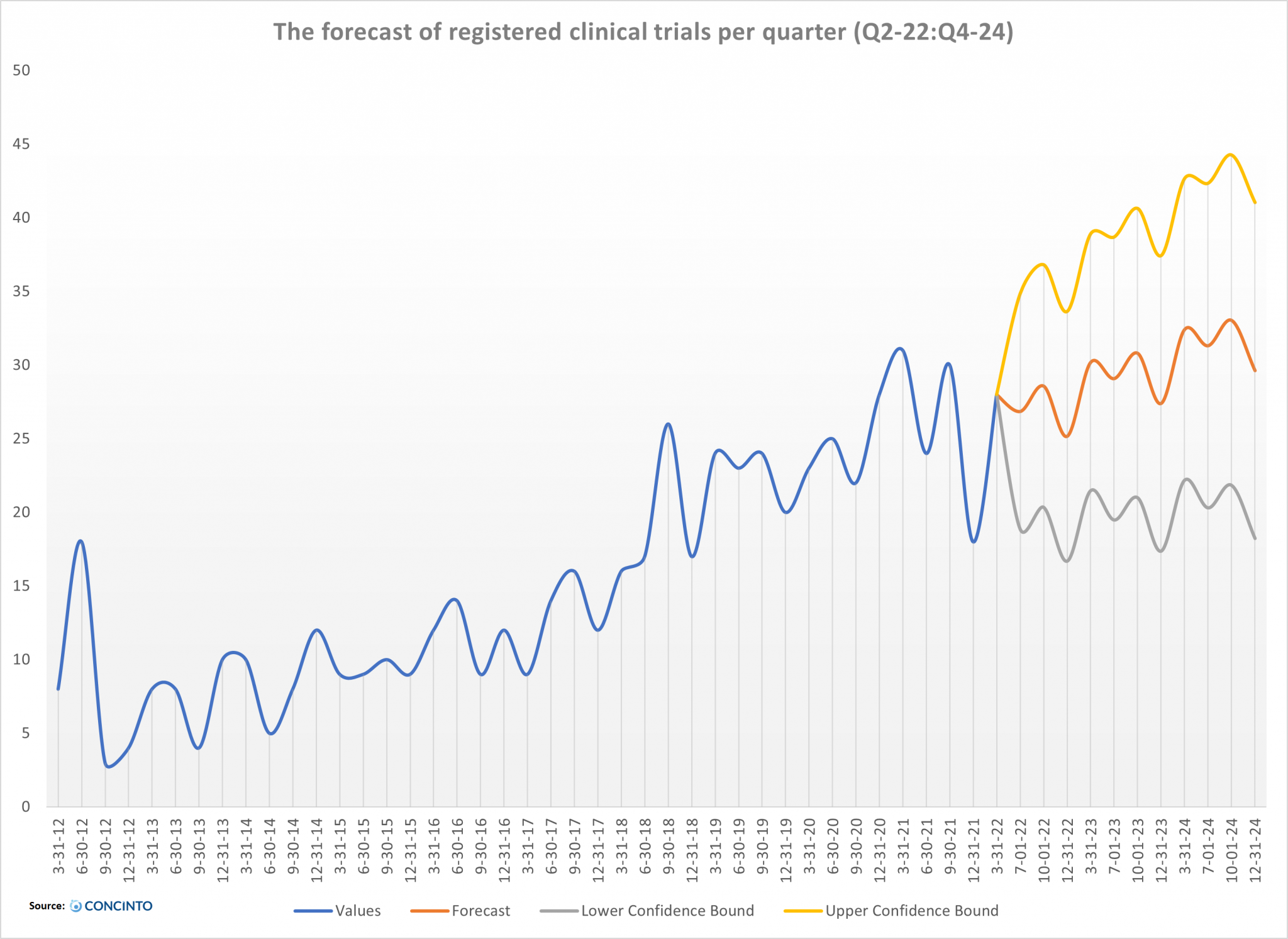
Although conducting clinical trials on cannabis and cannabinoids has been challenging for researchers and clinicians, the number of registered clinical trials has increased in recent years. Our data suggest that this trend will continue in the next ten quarters.
The following factors make this prediction a reality in the near future.
- Public interest: numerous polls in different countries have shown that the interest in cannabis for medical purposes has increased internationally. This shift in public opinion has changed the political calculus, and more politicians publicly support the legalization of cannabis.
- De-stigmatization of cannabis in medical communities: nowadays, more patients are openly talking about the use of cannabis for medical purposes with their healthcare practitioners. Moreover, more healthcare practitioners will see patients who use cannabinoids to manage their symptoms. These changes have transformed the clinicians’ attitude toward cannabis and cannabinoids and encouraged them to design clinical studies to answer their questions.
- The interest of pharmaceutical companies: the pharmaceutical companies are constantly looking for a new class of medications to address unmet medical needs. The Endocannabinoid System (ESC) is a less investigated target (because of the regulatory status and stigma) that can be the source of many novel medications. However, a good understanding of the physiology and pharmacology of the ECS is a requirement for a successful drug-development project that can only be accomplished by conducting proper clinical trials.
We have already started to see signs of these shifts. Regulatory reforms have started in several jurisdictions to open the pathways to ease access to medical cannabis. Regulatory authorities in major economies have developed frameworks for researching medical cannabis, which encourage pharmaceutical companies to invest more in preclinical and clinical research.
Unfavourable results or reports of serious adverse events will impact further research and weaken the interest of clinicians and commercial sectors. This scenario is not unlikely due to the ESC’s complexity and its effect on the function of several other receptors. Therefore, in our forecast, we have incorporated the possibility of a downward trend in the registration of clinical trials. We should keep in mind that the continuation of these upward trends depends on the result of current clinical trials.
Please contact us if you are interested to have more information about the Concinto clinical trial database.















