Systematic Review on Clinical Trials on Cannabis and Cannabinoids Our systematic review of the registered clinical trials on cannabis and…
William Paterson Cannabis Research Institute and Concinto Launch Medical Cannabis Education Program William Paterson University’s Cannabis Research Institute has launched two…
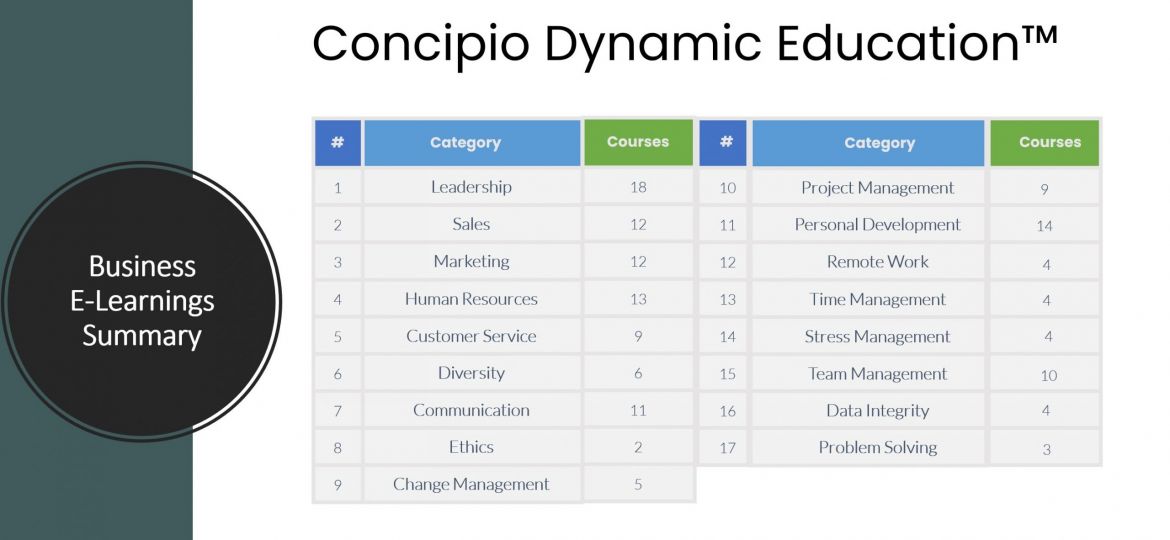
Concinto business e-learning Accelerate your team’s skills development with an online learning platform built to maximize learner engagement and long-term…
Concinto and William Paterson University signed an education collaboration agreement.
Concinto is a registered supplier on Scientist.com
Concinto introduces an accredited pharmacists certificate program.
BCPhA and Concinto sign a collaboration agreement.
Introducing Concipio, an interactive online healthcare education platform.
Concinto is now an accredited continuing education provider by CCCEP.
Finnish Food Authority has pulled CBD products from the market